Extraction of Metals
Contents
Extraction of Aluminium using Bauxite and electrolysis, Extraction of Iron using Hematite and blast furnace. Requirements for the reactions to take place.
Extracting Iron
From Text Books
Charge
Charge is added from the top of the furnace. It contains Iron Ore (Hematite), Limestone (CaCO3) and coke.
Reactions in Blast Furnace

Reactions in Blast Furnace
Here’s more about the reactions. Information of what is reduced and Oxidised and the type of reaction (Exothermic or Endothermic):

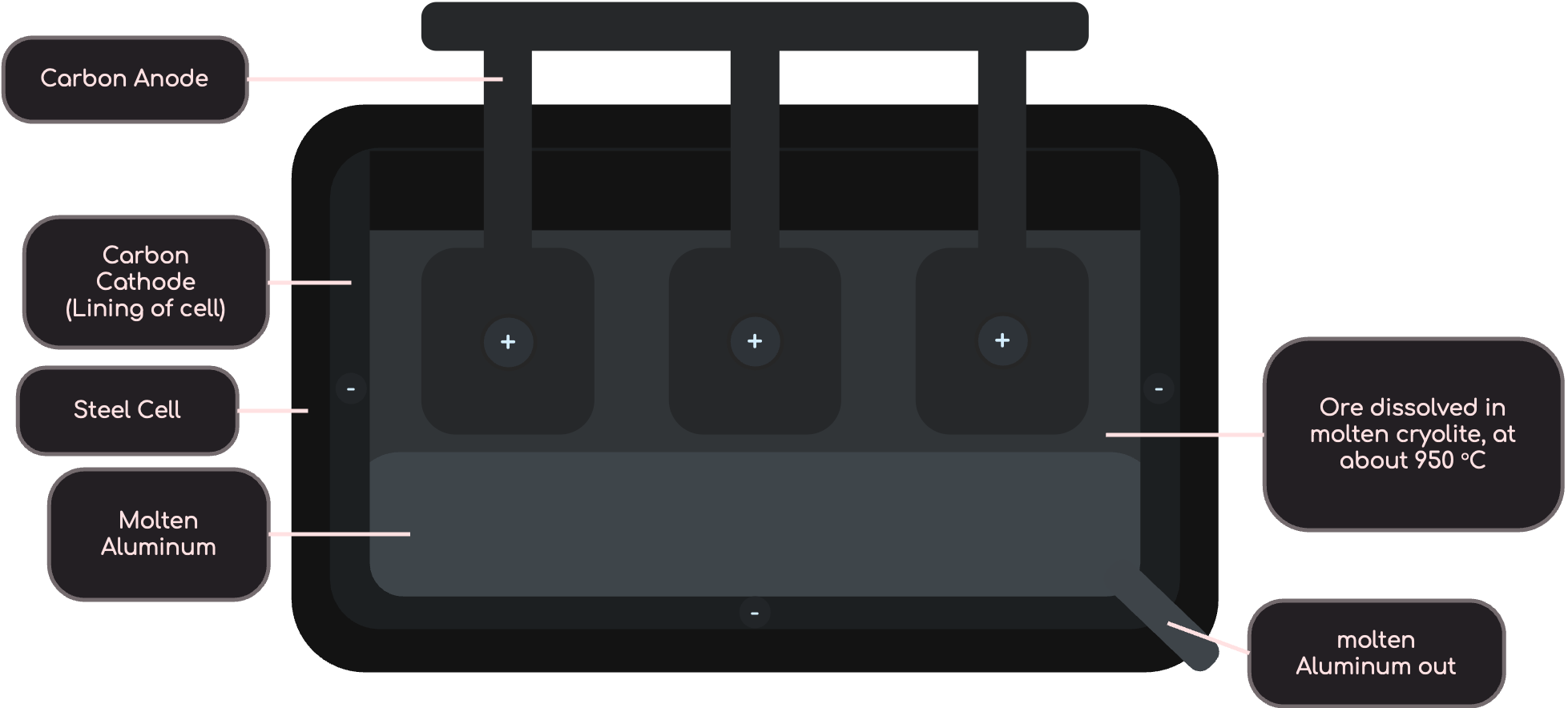
Extracting Aluminium
From Text Books
At the Cathode:
- 4Al3+ + 12e– ➜ 4Al (Reduction)
At the Anode:
- 6O2- ➜ 3O2 + 12e– (Oxidation)
- C + O2 ➜ CO2 (Oxidation of Carbon)
Overall Reaction:
2Al2O3 ➜ 4Al + 3O2 (Overall Reaction)
© Copyright 2024 - Made with Passion
