Periodic Table
Contents
Periodic Table, The Trends of Group I (Alkali Metals) and Group VII (Halogens), Transition Elements and their Properties.
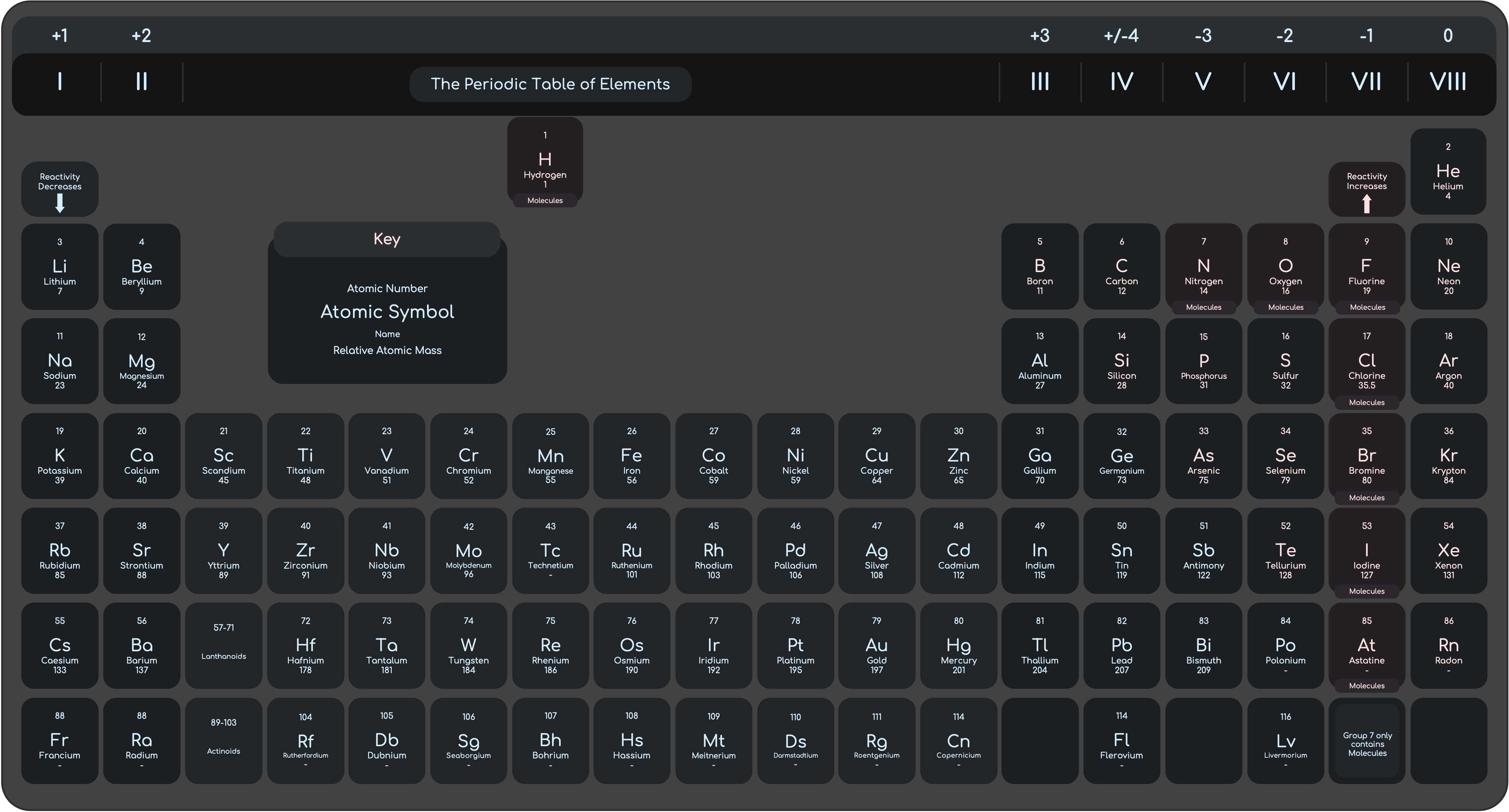
Group I (The Alkali Metals)
Alkali Metals are soft silvery metals. They only have 1 electron in the outer shell.
Physical Properties
Softer than most metals, Lighter than most metals, have low density and have low melting points.
- Density Increase down the group.
- Melting Point decreases down the group.
Chemical Properties
Alkali metals react vigorously with water, burst into flame with chlorine and can react with oxygen to form oxides. Alkali metals produce white solids after reactions, which can dissolve in water to form a colourless solution.
- Reactivity Increases down the group
From Text Books
Group VII (The Halogens)
Physical Properties
All halogens have different colour and all of them are poisonous. They form diatomic molecules.
- Density Increase down the group.
- Boiling Point Increase down the group.
COLOUR AND STATES
(Chlorine ➜ pale-yellow gas) (Bromine ➜ red-brown liquid) (Iodine ➜ grey-black solid)
Chemical Properties
- Reactivity Decreases down the group.
- They can react with metals and non-metals.
From Text Books
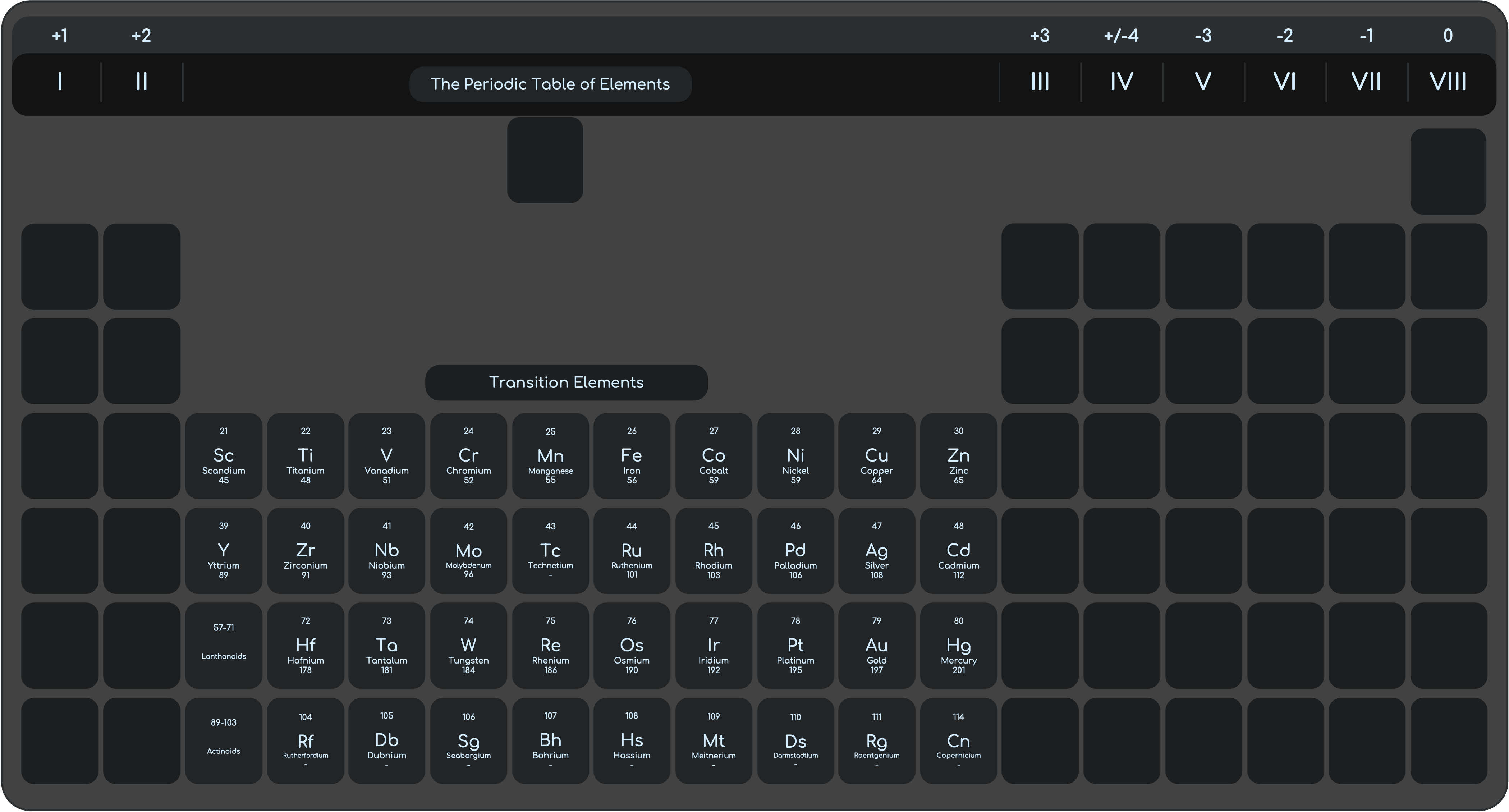
Properties of the Transition Elements
From Text Books
Physical Properties
- Higher Density when compared with Group I elements
- Higher Melting point when compared with Group I elements
- Hard and Strong unlike group I elements
- Good Conductors of heat and electricity
Chemical Properties
- Less reactive than Group 1 Elements
- Transition elements do not show clear trend in reactivity
- Most Transition elements form coloured compounds
- Transition elements and their compounds can act as catalysts
- Variable Oxidation States
© Copyright 2024 - Made with Passion
